Explain Differences Between Single Crystal and Polycrystalline Material
Single crystals were grown using an aluminium flux and had a consistently slightly larger lattice parameter than the. 6 Define the terms i Space lattice ii Unit cell iii Solid solution and monotectic solution.
As more and more atoms transfer themselves to this growing solid it becomes macroscopically large.

. A major difference between single crystals and polycrystals was not only the microstructure evolution at low equivalent strains but also the development of a stable micro-texture which is. 4 Explain about crystalline material. Crystalline structure is hard to produce and it is rare in nature in contrast to polycrystalline structure.
Crystalline structure is uniform and has no boundaries but polycrystalline structure differs from this. In a material that is polycrystalline the atomic pattern of individual crystals are usually in different mutual orien. Even at infinite length scales each atom is related to every other equivalent atom in the structure by translational symmetry.
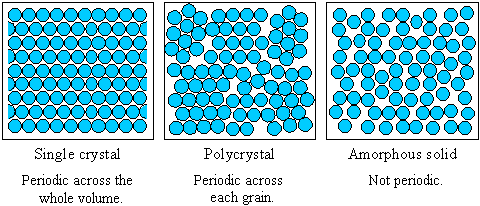
Single crystals have infinite periodicity polycrystals have local periodicity and amorphous solids and liquids have no long-range order. It is a collection of many atoms which is stable in the liquid. Single crystal solids are materials in which the crystal lattice of the entire sample is continuous and unbroken to the edges of the sample with no grain boundaries.
The main difference between single crystal and polycrystalline diamond lies in the structure of diamond particles which can actually be clearly seen under the microscope. The differences in the fragmentation of the microstructure and the micro-texture development between the single crystals and the polycrystalline aggregate were examined using EBSD. It does not have a continuous structure and it has boundaries between grains.
Single crystals have infinite periodicity polycrystals have local periodicity and amorphous solids and liquids have no long-range order. But there are also the crystal itself is a complete large grains the crystal is called single crystal crystal and. A polycrystalline UBe13 sample was made by arc-melting the constituents.
In terms of electrical properties polycrystalline silicon crystals have much lower conductivity than single crystal silicon and even electrical conductivity is very high. An ideal single crystal has an atomic structure that repeats periodically across its whole volume. The differences arise only in case of dense packing of single crystals as.
Materials containing crystal grains with dimensions below 100 nm are typically considered as nanocrystalline materials while materials containing crystal grains with dimensions above 100 nm are typically considered as polycrystalline materials. Feb 01 2020. If you have diffractometer with a.
Polycrystalline materials are solids that are composed of many crystallites of different size and orientation. This whole solid thus formed is a single crystal such as illustrated in Fig. Some of the crystal is composed of many small grains if the arrangement between the grains are no rules this is called polycrystalline crystal such as copper and iron.
The difference between single crystal and polycrystalline diamond. Although both abrasives are irregular polyhedral block particles the polycrystalline diamond grains are arranged in disorder. Polycrystals are composed of many small grains that have the same arrangement but are inconsistent in orientation.
Being made of a single crystal means that the internal atomic pattern is repeated without interruption save for the occasional crystal defect in 3 dimensions. The fundamental difference between single crystal polycrystalline and amorphous solids is the length scale over which the atoms are related to one another by translational symmetry periodicity or long-range order. A single crystal refers to a solid state in which molecules atoms or ions contained in the sample are regularly and periodically arranged in a three-dimensional space.
In simple words a poly-crystalline material is nothing but a huge collection of single crystals oriented randomly with respect to each other. Single crystal shows intense and sharp narrower peaks in XRD pattern but polycrystalline materials shows less intense and broad XRD peaks. C the ductility of the materials bonded in these ways.
An ideal single crystal has an atomic structure that repeats periodically across its whole volume. 5 Explain the procedure to find out the miller indices with an example. Each of these single crystals in a poly-crystalline material are called as grains and the corresponding boundary between two grains is called as a grain boundary.
Difference between single crystal and polycrystalline. Engineering materials usually contain many crystals ie they are polycrystalline. The key difference between crystalline and noncrystalline solids is that crystalline solids have an evenly distributed three-dimensional arrangement of atoms ions or molecules whereas non-crystalline solids do not have a consistent arrangement of particles.
The general physics of a microcrystal and a single crystal selected from the polycrystalline array is one and the same. Therefore the key difference between nanocrystalline and polycrystalline is that nanocrystalline materials. Answer 1 of 2.
Distinguish between single crystal material and polycrystalline material. Crystalline Solids and Non-crystalline Solids are the two main categories of solids that show some. Single crystals have infinite periodicity polycrystals have local periodicity and amorphous solids and liquids have no long-range order.
For example in terms of mechanical properties optical properties and thermal properties anisotropy it is less noticeable than single crystal silicon. Single crystal has a perfect orientation. Single crystals will show sharp Bragg spots whereas the polycrystal will show Debye rings in X-ray diffraction provided you diffractometer has a 2D detector.

Solved Questions 1 State The Difference Between Single Chegg Com

Polycrystalline Single Crystal And Non Crystalline Materials Pages 1 24 Flip Pdf Download Fliphtml5
No comments for "Explain Differences Between Single Crystal and Polycrystalline Material"
Post a Comment